Chemical treatments, as authorized in the 2022 EPA Construction General Permit, have proven to be effective tools in both erosion and sediment control applications. Properly deployed, such treatments have consistently delivered positive results in removing sediment from stormwater runoff and also in making soils less vulnerable to erosion.
Despite many benefits, chemical treatment often carries a negative connotation based on the perception that adding chemicals to our environment is inherently “bad.” Nearly every substance on the planet is a chemical or chemical compound including the building blocks of our bodies, the air we breathe, the food we eat and the materials we use to help clean our water.
The fact that nearly everything is a chemical or chemical compound does not mean that all chemicals are safe. Chemicals are commonly described as toxic versus nontoxic. Despite this “nontoxic” perception, all chemicals eventually reach a toxic amount. Paracelsus, a Renaissance physician, famously stated that everything is a poison and it is the dose, or the concentration of the chemical, that makes it toxic. For this reason, it is important to understand how to safely use chemicals for stormwater management and erosion control.
Safety is often determined by comparing the safe and effective amount to the toxic amount. For example, safe chemicals we consume daily include water and caffeine. If we consume or are exposed to a large enough amount, both water and caffeine can reach a harmful, even lethal level. However, the likelihood of reaching a toxic amount is very low which is why we label them as safe. The greater the space between helpful and hurtful, the safer the chemical. We refer to the distance, specifically the ratio, between the effective amount and the toxic amount as the margin of safety (MOS).
Chemicals Treatments
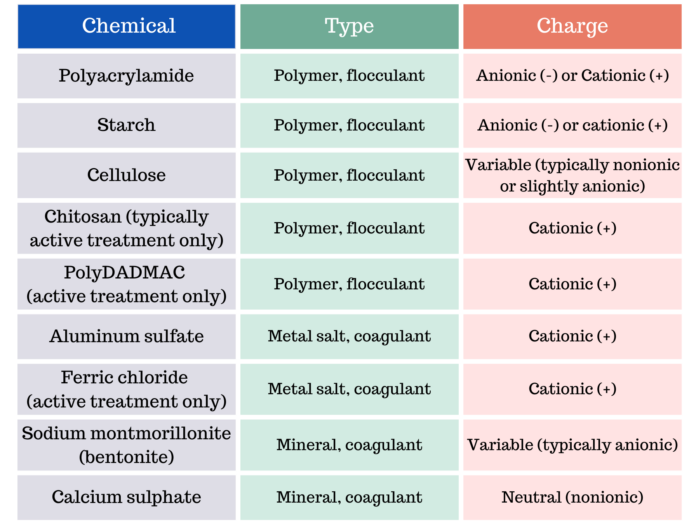
There are multiple commonly used chemicals for erosion and sediment control and stormwater treatment (Figure 1). Chemical charge is a primary characteristic that governs use. Anionic, or negatively charged, chemicals are generally considered safe for aquatic organisms by federal regulations in the USA and in almost every state. They have a relatively low toxicity, minimal restrictions and are used for passive environmental applications. In contrast, cationic, or positively charged chemicals have a high toxicity, require additional permits and are typically limited to controlled, active treatment systems.1
Tools for Chemical Treatment
The goal of all chemical treatment should be to use the lowest effective amount while being environmentally protective. Using too much treatment chemical is not more effective and can actually result in decreased effectiveness in addition to safety. To achieve the goal of safe and effective use, the application rate (effective amount) must be lower than the toxic amount (below a maximum allowable use rate). The further the application rate is below the safe and allowable use amount, the safer the treatment.
Application Rates
Product application rates are set primarily by manufacturers and are determined following testing and use. An application rate is used to ensure the product is applied in an amount that achieves desired results, for example, soil stabilization or water clarification. Application rates for water are typically measured in weight or volume per volume of water treated — lbs./gal or mg/L. Land applications are typically measured in weight or volume per area — lbs./acre or kg/hectare.
Maximum Allowable Use Rates
Following application rates does not guarantee safe use. Maximum allowable use rates are safe limits for chemical treatment. The maximum allowable use rate is the highest concentration of a substance that can be discharged without causing toxicity to aquatic organisms.2 Some states provide lists of approved chemical treatments and calculated maximum allowable use rates.3,5,6 Maryland Department of Environmental Quality and Wisconsin Department of Environmental Quality both use secondary and acute and chronic values to calculate maximum allowable safe use levels. North Carolina Department of Water Quality uses the most sensitive chronic values based on aquatic toxicity testing.8 However, calculating these values can be complicated2,6,7 and is not practical for the average user, therefore using available lists is ideal. However, since only three states in the USA currently provide these lists, not all available products are represented. If a product is not represented, there are other avenues to estimate maximum allowable use rates:
- Inquire with your local or state regulator.
- Contact the product manufacturer.
- Use the “no effects value” if available. With a basic understanding of toxicity information, using a no observed effects concentration (NOEC) is a viable option for chemical treatment users to estimate a protective, maximum allowable use rate.7,8
Toxicity Studies and the NOEC
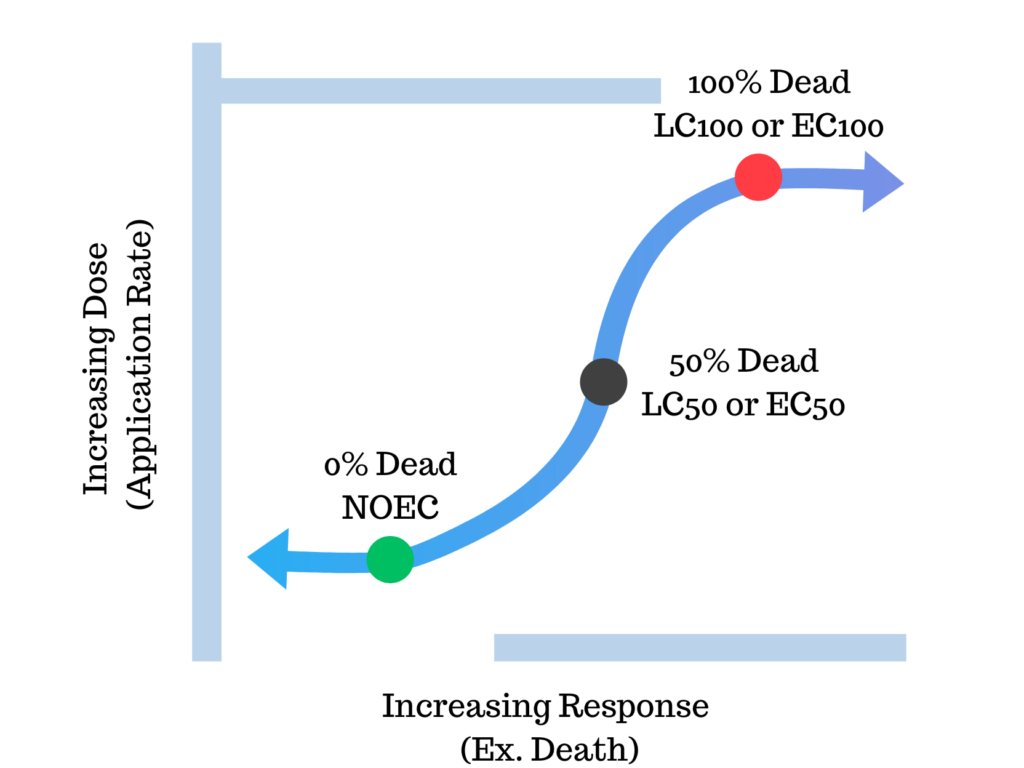
A toxicity study is a controlled, conservative laboratory test where sensitive organisms in their most sensitive life stage are exposed to a range of chemical concentrations. These tests can be done in a short period of time (acute; days) or a longer period (chronic; week(s)). Chronic tests are typically more sensitive. Results of these toxicity studies are calculated toxicity endpoints, or concentrations that negatively affected a percentage of organisms (Figure 2). Common endpoints include the EC50 or LC50 (effective concentration or lethal concentration where 50% of the organisms die or experience negative effects). Other important endpoints include the NOEC or NOAEL (no observed adverse effects level), this is the highest concentration where no effects were measured. Although the EC/LC50 is one of the most referenced toxicity endpoints, it is not a safe value to use as a maximum allowable use rate. The LC50 represents the amount of chemical that results in death of 50% of organisms. The NOEC represents 0% mortality. It is typically acceptable to use the chronic NOEC as a maximum use limit.7,8 Maryland Department of Environment suggests using “significantly lower concentration than the NOEC,”7 presumably to provide additional margin of safety and layers of protection.
If the NOEC is available, it can be found in toxicity study results (Figure 3) and summarized in Section 12 of safety data sheets (SDS). Manufacturers should be able to provide these documents upon request. When using an SDS, toxicity reports should still be reviewed to vet data accuracy since toxicity reports are written by third parties and SDS are written by manufacturers. State calculated maximum allowed use rates should be used if available. However, if guidance is not available, the NOEC provides a relatively simple means to estimate a safe use limit. Always follow federal, state and local regulations when applying any chemical treatment.
Margin of Safety
As previously stated, the MOS is the calculated ratio between the application rate and the maximum allowable use rate. The margin of safety is calculated by dividing the maximum allowable use rate by the application rate. Each individual product should be evaluated separately as even products with the same active ingredient (i.e. anionic polyacrylamide, chitosan acetate, sodium montmorillonite, etc.) may have variable toxicities, maximum allowable use rates and suggested application rates. Chemicals with little or no MOS (~one or less) should be limited to active treatment systems. Anionic chemicals with higher MOS can typically be used in passive, semi-passive and active treatment. Although there is no set MOS value that defines a safe chemical, the larger the margin the better. A product with a MOS of five and application rate of 100 pounds (45.4 kg) could safely use up to 500 pounds (227 kg) and remain below the safe maximum allowable use rate. An important thing to ensure is that the same units are being compared between application rates and maximum allowable use rates. We often see application rates listed as lbs./gallon while toxicity values and maximum allowable use rates are listed in mg/L. If assistance is needed, manufacturers should be able to convert the values, or search engines like Google can perform conversions by typing convert mg/L to lbs./gallons in the search bar.
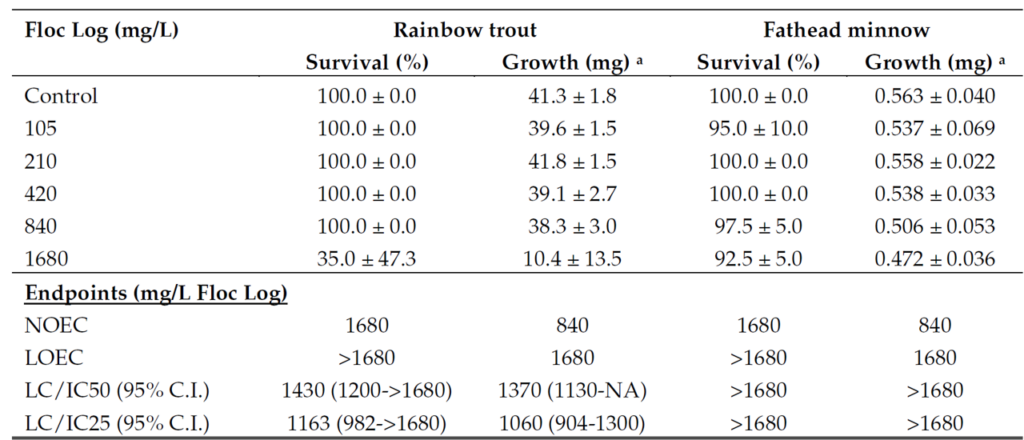
Example 1. Anionic polyacrylamide flocculant log (706b Floc Log)
Suggested Application Rate: 2 to 5 mg/L (16.6-41.5 lbs./1 million gallons water).
Maximum Allowable Use Rate: 42 mg/L (348.6 lbs./1 million gallons water).3
Margin of Safety (MOS): 42 mg/L ÷ 2 to 5 mg/L = 21 to 8.4.
Example 2. Bentonite based
granular (FLOC)
Suggested Application Rate: 180 mg/L (1,494 lbs./1 million gallons water).10
Maximum Allowable Use Rate: 650 mg/L (5,395 lbs./1 million gallons water).4
Margin of Safety (MOS): 650 mg/L ÷
180 mg/L = 3.6.
Example 3. 1% Chitosan acetate liquid (1% Liquifloc)*
Suggested Application Rate: 100 to 300 mg/L (834-2,503 lbs./1 million gallons water).10,11
Maximum Allowable Use Rate: 121 mg/L (1,010 lbs./1 million gallons water).9
Margin of Safety (MOS): 121 mg/L ÷ 30 to 300 mg/L = 1.2 to 0.4.
*Diluted products need extra care when reviewing and calculating to ensure that application rates and maximum allowable use rates are referring to the same solution and product. Diluted solutions will have different toxicity values and application rates than concentrated active ingredients (i.e. 100% chitosan acetate vs. 1, 2 or 3% liquid solutions).
Steps to Safe Dosing
By comparing the application rate, maximum allowable use rate and the margin of safety, we can make informed decisions on which chemicals to apply and in what amount. These three values provide a relatively simple method to wade through hundreds of available products with multiple active ingredients. When used correctly, chemicals can help solve many challenges faced in managing water quality and erosion and sediment control. The use of existing tools combined with the concepts presented in this article should serve to provide conservative guidance for safe and effective chemical treatment applications to control erosion and clarify turbid water while protecting valuable land and water resources.
References
- EPA 2022. National Pollutant Discharge Elimination System (NPDES) Construction General Permit (CGP) for Stormwater Discharges from Construction Activities. United States Environmental Protection Agency. February 17, 2022.
- Wisconsin Department of Natural Resources. Additives. 2024. https://dnr.wisconsin.gov/topic/Wastewater/Additives.html.
- MDE 2024. MDE Flocculant: Chemical Additives Forms and Guidance, Approved List. Maryland Department of Environmental Protection 2024. https://mde.maryland.gov/programs/permits/WaterManagementPermits/Pages/MDFlocs.aspx.
- NC 2024. North Carolina DWR List of Approved PAMS/Flocculants. North Carolina Division of Water Resources. April 30, 2024. https://files.nc.gov/ncdeq/Water+Quality/Environmental+Sciences/ATU/PAM8_30_18.pdf.
- Wisconsin Department of Natural Resources. 2023. Previously Reviewed Additives List. https://dnr.wisconsin.gov/topic/Wastewater/Additives.html.
- EPA 1985. Guidelines for Deriving Numerical National Water Quality Criteria for the Protection Of Aquatic Organisms and Their Uses. Environmental Protection Agency. https://www.epa.gov/sites/default/files/2016-02/documents/guidelines-water-quality-
- criteria.pdf.
- MDE 2019. Procedures for Review of Chemical Additives for Sediment Control. Maryland Department of Environmental Protection. April 30, 2019.
- https://mde.maryland.gov/programs/permits/WaterManagementPermits/Documents/Chemical-
- Additives/Additive-Review-Procedure.pdf.
- NCDWR. June and July 2024. Email correspondence with Aquatic Toxicology Branch, Water Quality Division, North Carolina Department of Natural Resources. Topic: How use levels are calculated in published North Carolina DWR List of Approved PAMS/Flocculants. WADOE 2017. Use Designations for Erosion and Sediment Control for Chitosan-Enhanced Sand Filtration using 1% HaloKlear® LiquiFloc™ chitosan acetate solution. https://fortress.wa.gov/
- ecy/ezshare/wq/tape/use_designations/LIQUIfloc1PCTguld.pdf.
- Kazaz, Billur. 2022. Improvements in Construction Stormwater Treatment using Flocculants. Dissertation, Auburn University.
- Macpherson, John. Storm-Klear™ (Chitosan) Toxicity and Applications Construction Stormwater Treatment. October 2004.
- https://www.waterboards.ca.gov/water_
- issues/programs/stormwater/docs/advtreat/naturalsitesolutions.pdf.
About the Expert
Kyla Wood, Ph.D., is a biologist and aquatic toxicologist with a passion for clean water and applying science to find real world solutions. She is chief science officer for Applied Polymer Systems, Inc. and leads APS’s research and development, training and regulatory operations.