Impounded lakes provide essential water supply, flood control and hydropower to cities worldwide, but they also inundate habitat, lose water by seepage and evapotranspiration, and capture sediment that takes up storage and would otherwise replenish downstream environments. Rivers and shore areas input most of the sediment trapped in built reservoirs, and unconsolidated deposits are especially vulnerable to wave erosion in shore areas.
Some forms of natural vegetation such as buttonbush (Cephalanthus occidentalis L. [Rubiaceae]) can bind shore sediment while providing a habitat for birds and other animals. Often found in floodplains and riparian zones, buttonbush naturally occupies parts of southeastern Canada and the eastern United States, extending westward to eastern Texas, Oklahoma, Kansas, Iowa, Wisconsin and Michigan.1 Scattered populations of buttonbush are also found in Arizona, New Mexico, southern California and northern Mexico.
Common buttonbush is a hardy shrub with an irregular crown, spheres of white flowers and button-shaped fruit.2 Buttonbush typically blooms in summer, produces fruit in early fall and reaches a height of approximately 3 m (10 feet) at maturity.3,4 Birds consume buttonbush seeds, mallards (Anas platyrhynchos) eat its fruit, wood ducks (Aix sponsa) use it for nests, and deer browse its foliage.5,6,7,8 Buttonbush also provides nectar to bees, butterflies, moths and hummingbirds (Trochilidae).9,10 Three types of moths, the hydrangea sphinx (Darapsa versicolor), titan sphinx (Aellopos titan) and royal walnut moth (Citheronia regalis), use buttonbush as a larval host.11
Buttonbush has no major insect, pest or disease problems.12 Along lake shores, buttonbush can form dense stands, with swollen bases that anchor shrubs and bind sediment.13,14 Through adaptation, buttonbush also tolerates disturbance from humans. A study along a segment of lake shore in north-central Texas explored adaptive strategies to wave erosion, pruning and mowing.
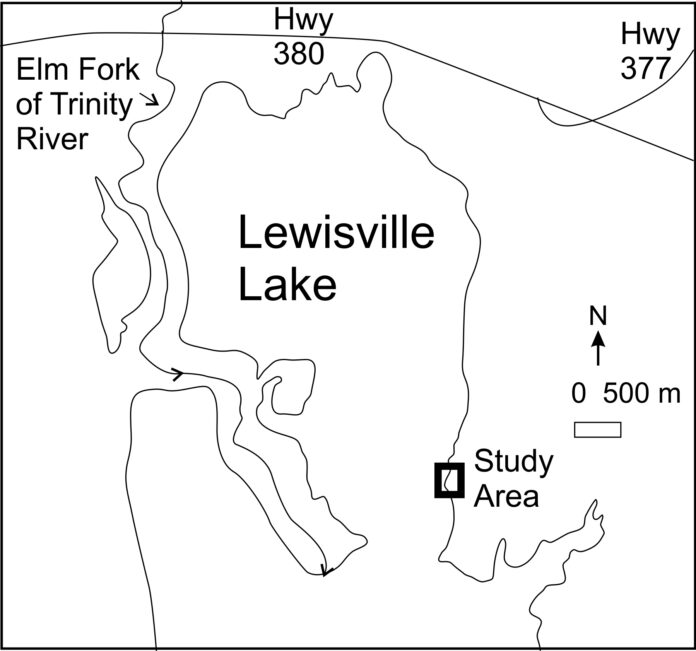
Study Area
The study area is located near the northern edge of Lewisville Lake in north-central Texas (Figure 1). This large, multi-use reservoir is impounded by an earthen dam located 16 km (10 miles) south of the study area. Lewisville Lake provides water to local cities and water authorities, controls flooding and supports recreation such as fishing and boating. The lake’s maximum normal operating level (conservation pool) is 159.1 m (522 feet) above mean sea level.15 The lake tends to rise above and fall below conservation pool each year, except during drought. Winds create waves that impact the study area from all directions, with a relatively strong SSE mode over a typical year.
Along the shoreline, a thin blanket of Quaternary alluvium (terrace) overlies bedrock of the Upper Cretaceous Woodbine Formation. In the study area, the Woodbine Formation consists predominantly of loosely cemented sandstone and clay. Overlying terrace deposits comprise sand, gravel and clay. Soil has formed and remains intact, but is eroding, on terrace deposits at the back of the shore.




Observations
Buttonbush was observed and photographed along a representative segment of developed shore. Photographs were taken on 22 May 2023, when lake elevation was 159.4 m (522.7 ft) above mean sea level.16 Buttonbush was growing in a narrow elevation range, within approximately half a meter (1.6 feet) of conservation pool. Shrubs were anchored into both alluvial deposits and the underlying Woodbine Formation.
Shrub heights ranged from less than 1 m (3 feet) to approximately 3 m (10 feet). At ground level, plant diameters ranged from less than 1 cm (0.4 inch) to approximately 10 cm (3.9 inches). Based on growth rings of cut shrubs and associated diameters, shrubs ranged from less than one year to approximately 20 years old. Having survived frequent wave action, and in some cases pruning and mowing from adjacent landowners, mature shrubs were binding sediment and providing habitat to various fauna.
Three adaptive strategies were observed: regrowth below pruned branches, root anchoring and seed dispersal. New branches typically emerged within a few centimeters (1 inch) of the cut level on a branch, often taking a similar trajectory to the pruned branch (Figure 2). In some cases, entire shrubs lopped off at ground level sprouted new stems around the perimeter of the stump.
Several shrubs had been undermined by wave erosion but were still thriving (Figure 3). Typically, undermined shrubs adapted by hardening exposed roots into three or more supporting legs connected at the top by the exposed root ball. Each supporting leg developed a new root ball beneath the ground for stability.
Exposed root balls document previous ground levels, thereby facilitating erosion rate estimates. For example, an approximately five-year-old, partially submerged shrub in one location of the study site indicates about 50 cm (19.6 inches) of denudation over five years, or 10 cm (3.9 inches) per year. Concurrently, the backshore terrace retreated about 1 m (3 feet) over the five-year period. Such erosion estimates can be useful to planners, to guide or prioritize restoration efforts that might involve additional plantings or other stabilizing structures.
Seed dispersal was the most important adaptive strategy. Nearly 300 seeds were counted in a single fruit ball in the fall of 2022. Dispersed seeds were examined on a contrasting, white paper background and appeared healthy: of characteristic shape, size and color; plump, full and fresh; devoid of shriveling, mottling or other damage; and free from pests and disease. Moreover, each mature shrub typically produced 20 or more fruit balls, thus releasing thousands of seeds per shrub. With such volume, even low survival rates result in considerable propagation. Seeds showed propensity to grow in Bermuda grass that invaded the study area, as well as in reworked sediment above hummocky bedrock.
Grass cover protected settled seeds from water and wind. Seedlings and shrubs grew in grass along the edge of the terrace and below the terrace (Figure 4). Typically, the edge of the terrace escaped mowing, but not less frequent string trimming. At the terrace edge, up to 20 seedlings were growing in a square meter of turf grass. In some cases, property owners trimmed Bermuda grass around buttonbush shrubs, preserving them as ornamental features for aesthetic value.
Seeds also settled into micro-depressions between hummocks formed by concretions in bedrock (Figure 5). Concretions form inside sediment shortly after deposition, as minerals precipitate from solution, sometimes as successive layers around a nucleus such as a granule, plant remnant or small shell.17 Between exposed concretions, which are relatively resistant to erosion, sediment and moisture accumulated to nurture seeds and support plants. Some plants were growing inside broken concretions. Eventually, roots propagated through cracks inside concretions, or into sediment between concretions, and anchored into more stable underlying deposits.
Conclusion
Buttonbush in the area is hardy, surviving not only frequent wave activity, but also indiscriminate pruning and mowing from adjacent property owners. Key adaptive strategies that include root anchoring, branch regrowth and seed dispersal, enable buttonbush to thrive in this setting. Buttonbush is valuable, providing habitat to various animals, stabilizing the shoreline, and enabling estimates of erosion rates to inform shoreline stabilization efforts.
References
- LBJWC (Lady Bird Johnson Wildflower Center). 2023. Plant Database. Lady Bird Johnson Wildflower Center, Austin, Texas. Available from: https://www.wildflower.org/plants/result.php?id_plant=ceoc2.
- LBJWC 2023.
- Polomski, R.F. and Feder, B.H. 2020. Common Buttonbush. Factsheet HGIC 1098. Clemson Cooperative Extension, Clemson University, Clemson, South Carolina. Available from: https://hgic.clemson.edu/factsheet/common-buttonbush/.
- Wennerberg, S. 2004. Common Buttonbush (Cephalanthus occidentalis L.). Natural Resources Conservation Service, United States Department of Agriculture. Available from: https://plants.usda.gov/Document Library/plantguide/pdf/pg_ceoc2.pdf.
- Little, E.L. 1980. The Audubon Society Field Guide to North American Trees: Eastern Region. Knopf, New York.
- Niering, W.A. and Olmstead, N.C. 1985. The Audubon Society Field Guide to North American Wildflowers: Eastern Region. Knopf, New York.
- Parr, D.E., Scott, M.D. and Kennedy, D.D. 1979. Autumn movements and habitat use by wood ducks in southern Illinois. Journal of Wildlife Management, 43(1), 102-108.
- Wennerberg 2004.
- Snyder, S.A. 1991. Index of Species Information. Fire Effects Information System, U.S. Department of Agriculture. Available from: https://www.fs.fed.us/database/feis/plants/shrub/cepocc/all.html.
- Wennerberg 2004.
- Wheeler, J. 2017. Planting for Pollinators: Buttonbush. Xerces Society for Invertebrate Conservation, Portland, Oregon. Available from: https://xerces.org/blog/planting-for-pollinators-button-bush#:~:text=In%20addition%20to%20its%20attractiveness,walnut%20moth%20(Citheronia%20regalis).
- Polomski and Feder 2020.
- Polomski and Feder 2020.
- Wennerberg 2004.
- USACE (U.S. Army Corps of Engineers). 2023. Lewisville Lake. Lewisville Lake Project Office, U.S. Army Corps of Engineers, Lewisville, Texas. Available from: https://www.swf-wc.usace.army.mil/lewisville/.
- USGS (U.S. Geological Survey). 2023. Lewisville Lake near Lewisville, Texas. Available from: https://waterdata.usgs.gov/monitoring-location/08052800/#parameterCode=62614&timeSeriesId=139931&start DT=2022-01-01&endDT=2022-12-31.
- Todd, J.E. 1903. Concretions and their geological effects. Bulletin of the Geological Society of America, 14, 353-368.
About the Expert
Paul F. Hudak, Ph.D, is a professor in the Department of Geography and the Environment at the University of North Texas.