Flocculants and coagulants are vital tools used globally to improve water quality by removing harmful contaminants such as sediment, metals and excess nutrients. Industries such as stormwater, mining, agriculture, construction, drinking water, wastewater, and pond and lake management all rely on chemical flocculants and/or coagulants. Chemical treatments make it possible to remove suspended and dissolved contaminants efficiently and effectively from water to make it safe for consumption, use and/or discharge back to the environment (Figure 1).
With increasing population, industry and construction activity, a greater need for chemical treatments has led to increases in production and use globally and created interest and concern over chemical source and environmental fate. In a perfect world, available water treatment chemicals would marry effectiveness, safety (low toxicity) and sustainability (rapid degradation and renewable sourcing). Like most things, however, each treatment comes with advantages and disadvantages. The goal is to combat water quality issues in the most environmentally responsible ways possible by effective use of existing technologies and continued development of new technologies. Multiple existing treatments already offer effective and safe solutions for water quality issues. However, recent work has shown that combining specific types of existing polymers together can create new, more sustainable solutions.
Common Flocculants and Coagulants: Important Properties
Common flocculants and coagulants used for stormwater treatment include polyacrylamides (polymer flocculant), plant and animal-based biopolymers (starch/ cellulose, chitosan), and metal salt coagulants like alum. Flocculants and coagulants are both able to effectively treat water by separating suspended solids and contaminants from water. Coagulants work by destabilizing the charge on colloidal, suspended particles so they can settle out of solution. Flocculants enable particles to bind together, forming larger, heavier particles or “flocs” that rapidly settle.
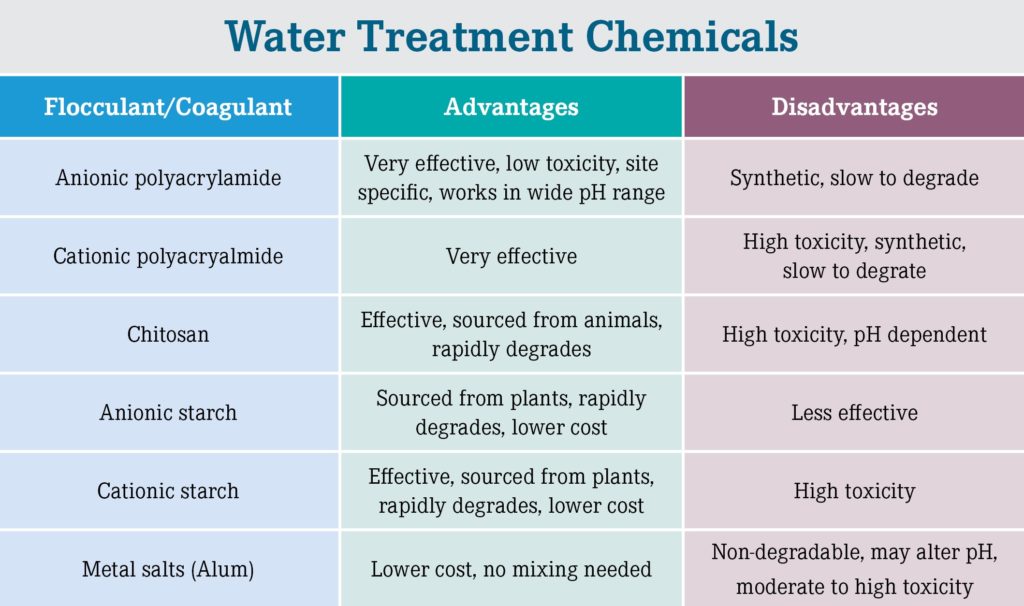
Although each base material looks similar (white granule, flake or powder), they all have different properties that can affect their utility and desirability for specific applications (Figure 2). These important properties include effectiveness, toxicity, source material and environmental fate and are dependent on several factors.
Effectiveness of polymer flocculants is dependent on the molecule size (weight), charge (+ vs. -), and charge density (how much charge is on a polymer chain). Synthetic polymers can be any size but are generally much larger than natural polymers. Larger polymers can bind more particles, form larger floc, and are typically highly effective at low application rates. Their larger size also allows them to bind contaminants regardless of charge. For example, anionic and nonionic polymers can effectively and efficiently remove anionic clay particles despite both having negative charges. Positive (cationic) chemicals can bind directly to negatively charged particles like clay and sediment and are often very effective. However, the tradeoff is toxicity to gilled organisms as positive chemicals bind to negatively charged gill surfaces and cause suffocation.
Toxicity is mainly determined by flocculant charge: cationic (+) vs. anionic (-). Natural and synthetic cationic flocculants are effective for water treatment but carry a high toxicity to aquatic organisms (Liber et al. 2005, WA DOE 2019). Anionic polymers have 10-100x lower toxicity than cationic polymers (with the same effective rate) and are therefore the first choice of states regulatory agencies for environmental applications and typically do not require special permits for use. Biodegradability (environmental fate) is typically based on the source material such as synthetic vs. plant/ animal and its chemical composition and structure. Natural polymers are more easily degraded by bacteria and other microorganisms resulting in degradation in days to weeks, compared to months to years for large, synthetic polymers.
Common Flocculants and Coagulants: The Key Players
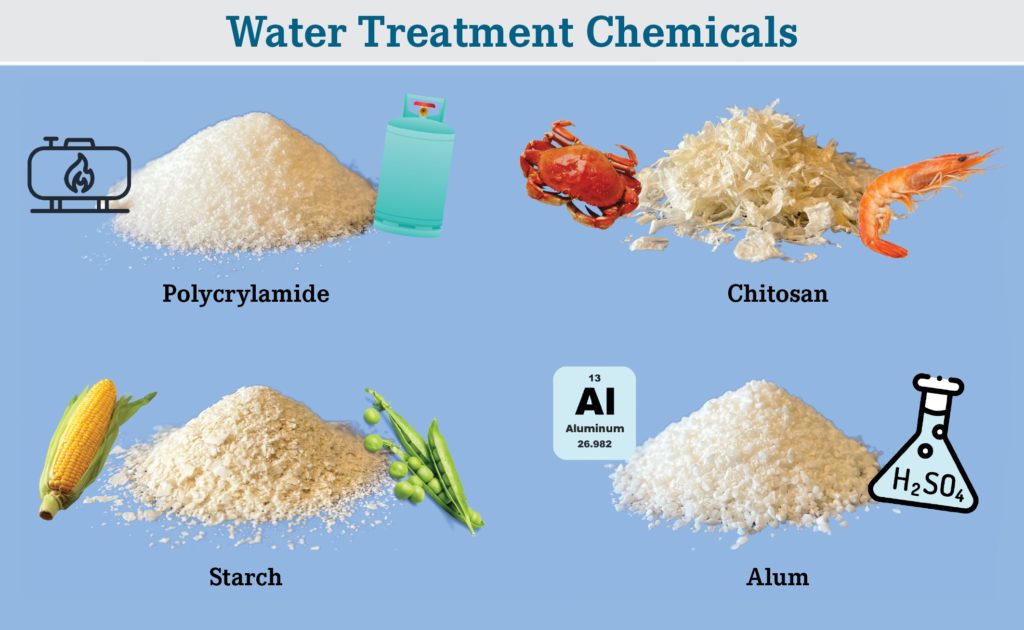
There are four common flocculants and coagulants (Figure 3).
- Polyacrylamides (PAM)
Large, anionic PAMs are the most widely used polymers for stormwater treatment. Widespread use is due to low toxicity, high effectiveness and ready availability. PAM has also been thoroughly utilized and studied with over 70 years of research and use as a soil amendment and more than 50 years as a water treatment chemical (Entry et al. 2002). Despite PAMs widespread use some states do not promote or allow the use of PAM over slow biodegradability concerns.
- Starch
Starch is one of the most widely used and available biopolymers as it is fully biodegradable and derived from commonly available plants such as corn, soybeans, potatoes, wheat and tapioca. Anionic starch flocculants carry a low aquatic toxicity but are not commonly used for sediment and metal removal since they are less effective (Asharuddin et al. 2021) and require a dosage 2 to 10 times greater than PAM (Wang 2018, Lapointe & Barbeau 2015).
- Chitosan
Chitosan is a polysaccharide derived from shells of crustaceans. It has multiple uses in water treatment, pharmaceutical and pesticide industries. It is readily biodegradable and naturally sourced; however, it is cationic and has a high toxicity to aquatic organisms (like cationic PAM and starch). Its narrow margin of safety for aquatic organisms is why the U.S. Environmental Protection Agency and most states do not allow or require a special permit for use in environmental applications (USEPA 2022, FLDEP 2018, Illinois Urban Manual 2011, EGLE, EGLE 2021).
- Metal Salts (Alum)
Coagulants like aluminum and other metal salts are positively charged chemicals (not polymers) that neutralize the negative charge on colloidal particles and allow them to slowly settle out of solution. They are typically low cost and do not require mixing like polymer flocculants. However, many carry a moderate to high toxicity and they do not break down, so there are concerns of environmental fate and build up in the environment – particularly in sediment.
Benefits of Combining Treatment Chemicals
Combining existing flocculants together such as anionic starch and PAM, has created new products that compound the advantages of the materials while minimizing the disadvantages.
Anionic PAMs are widely used and provide effective treatment with a high safety margin for aquatic organisms, albeit slow degradation. Some regulatory agencies and flocculant users look at chemical fate first and select naturally sourced and rapidly degrading products – in turn opting for cationic chemicals with higher toxicity, or less effective anionic flocculants such as starch. Combining synthetic and plant based anionic polymers may provide a solution to this problem, so we do not sacrifice safety or efficacy for degradability.
Blending anionic starch flocculants with anionic polyacrylamides can provide a low toxicity, high-margin-of-safety material that can effectively remove harmful contaminants from water while doubling the use of plant-based flocculants. These blended flocculants produce comparable results to PAM (Iwinski 2021) while including benefits such as increased biodegradability, sustainable sourcing (plants) and potentially reduced cost as PAM prices are subject to oil and gas availability and fluctuations.
Conclusion
We live in a world of relative risk and all advantages also come with disadvantages. All chemicals discussed in this article have inherent benefits of removing known and acutely harmful substances from water. As population and water quality concerns continue to increase, use of flocculants that strive to balance safety and sustainability will help us meet industry needs while working to preserve our natural resources.
References
Asharuddin, S., Othman, N., Altowayti, W., Bakar, N., & A. Hassan. 2021. Recent advancement in starch modification and its application as water treatment agent. Environmental Technology and Innovation 23.
EGLE. Michigan Department of Environment, Great Lakes, and Energy. Polyacrylamide, BMP Technical Data Sheet. https://www.michigan.gov/-/media/Project/Websites/egle/Documents/Programs/WRD/NPS/Tech/BMP/bmp-polyacrylamide.pdf?rev=704e168ca40a4ccb8649fd7fe4da068a.
EGLE 2021. Michigan Department of Environment, Great Lakes, and Energy. Polyacrylamide Products and Soil Erosion and Sedimentation Control Technical Guidance. https://www.michigan.gov/-/media/Project/Websites/egle/Documents/Programs/WRD/Storm-Water-SESC/polyacrylamide-products-technical-guidance.pdf?rev=a82bf9c21cc8434183da52892e1f3f88.
FLDEP 2018. Florida Department of Environmental Protection Florida Stormwater Erosion and Sedimentation Control Inspectors Manual, 2018. https://publicfiles.dep.state.fl.us/DEAR/DEARweb/Stormwater_training/Manual/FSESCI%20TIER%20I%20Manual%20100318.pdf.
Illinois Urban Manual Practice Standard, 2011. Polyacrylamide (PAM) for Turbidity. https://illinoisurbanmanual.org/wp-content/uploads/2018/08/PAM-for-Turbidity-Reduction-and-Sediment-Control-1.pdf.
Iwinski, K. 2022. Biopolymers and PAM: Evaluation of Novel Bio-Blend Polymer Logs for Removal of Suspended Sediment. IECA International Conference Paper, Minneapolis, MN.
Lapointe, M. & B. Barbeau. 2015. Evaluation of activated starch as an alternative to polyacrylamide polymers for drinking water flocculation. Journal of Water Supply: Research and Technology 64.3 (333-343).
Liber, K., L. Weber, & C. Levesque. 2005. Sublethal toxicity of two wastewater treatment polymers to lake trout fry (Salvelinus namaycush). Chemosphere 61 (1123-1133).
USEPA 2022. United States Environmental Protection Agency Construction General Permit. Appendix J – Suggested Format for Request for Chemical Treatment (updated 02/22). https://www.epa.gov/system/files/documents/2022-01/2022-cgp-final-appendix-j-request-for-chemical-treatment.pdf.
WA DOE (revised 2019). 2019 Stormwater Manual for Western Washington. https://fortress.wa.gov/ecy/ezshare/wq/Permits/Flare/2019SWMMWW/Content/Resources/DocsForDownload/2019SWMMWW.pdf.
Wang, D. 2018. Activated starch as an alternative to polyacrylamide-based polymers for in-line filtration of low turbidity source water. Journal of Water Supply: Research and Technology 67.5 (467-471).
About the Expert Kyla Iwinski-Wood, Ph.D., is an aquatic toxicologist with a passion for clean water and applying science to find real-world solutions. She is director of research and development for Applied Polymer Systems, Inc.